Abstract
Introduction: Recently, there have been significant advances in the number of therapies available for patients with multiple myeloma (MM). However, there is a continued need to identify and develop effective treatment strategies for patients with relapsed MM. The final progression-free survival (PFS) analysis of the Phase 3 IKEMA study (NCT03275285), performed 2 years after the prespecified interim analysis, confirmed that isatuximab (Isa) + carfilzomib (K) and dexamethasone (d) (Isa-Kd) significantly improved PFS compared with Kd in patients with relapsed MM (hazard ratio [HR] 0.58; 95.4% confidence interval [CI], 0.42-0.79), with clinically meaningful increases in minimal residual disease negativity (MRD-) (33.5% vs 15.4%) and complete response (CR) (44.1% vs 28.5%) rates in the intent-to-treat population, and a manageable safety profile. Here, we present updated efficacy and safety results from IKEMA by number of prior lines of therapy (1 vs >1).
Methods: Patients with 1-3 prior lines of therapy were randomized 3:2 to receive Isa-Kd (n=179) or Kd (n=123). Treatment was given until progressive disease or unacceptable toxicity. These updated, longer-term data are based on a prespecified final PFS analysis (primary endpoint) of IKEMA at 159 PFS events. Key secondary endpoints included MRD- rate and CR rate (using the HYDRASHIFT Isa immunofixation assay). MRD- and CR rate and safety were also updated.
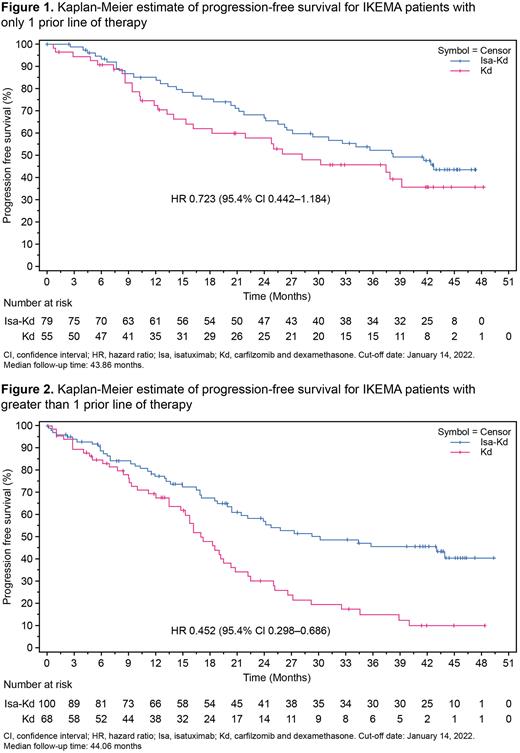
Results: Of the 302 randomized patients, 134 (44.4%; 79 Isa-Kd, 55 Kd) had received 1 prior line of therapy, and 168 (55.6%; 100 Isa-Kd, 68 Kd) had received >1 prior line of therapy. At a cut-off date of January 14, 2022 (median follow up of 44 months in each subgroup), PFS was improved with Isa-Kd vs Kd across both subgroups, consistent with the primary endpoint in the intent-to-treat population. Among patients who received 1 prior line of therapy, median PFS was 38.24 months with Isa-Kd and 28.19 months with Kd (unstratified HR 0.723; 95.4% CI 0.442-1.184; P= .0983; Figure 1). In patients who received >1 prior line of therapy, median PFS was 29.21 months with Isa-Kd and 16.99 months with Kd (unstratified HR 0.452; 95.4% CI 0.298-0.686; P< .0001; Figure 2). Depth of response improved with the addition of Isa to Kd across both subgroups. More patients achieved CR with Isa-Kd than with Kd (48.1% vs 38.2%, 1 prior line; 41.0% vs 20.6%, >1 prior line). The addition of Isa to Kd also yielded higher rates of both MRD- (39.2% vs 21.8%, 1 prior line; 29.0% vs 10.3%, >1 prior line) and MRD- and CR (32.9% vs 16.4%, 1 prior line; 21.0% vs 8.8%, >1 prior line). Nearly all patients experienced at least one treatment-emergent adverse event (TEAE; range of 97.1% to 99.0% across subgroups and treatment arms). The frequency of Grade ≥3 TEAEs was generally similar between the subgroups (83.3% [Isa-Kd] and 72.2% [Kd], 1 prior line; 83.8% [Isa-Kd] and 73.5% [Kd], >1 prior line). Serious TEAEs occurred in 66.7% vs 51.9% of patients (1 prior line subgroup) and 72.7% vs 66.2% of patients (>1 prior line subgroup) with Isa-Kd vs Kd, respectively. The percentage of patients with TEAEs leading to treatment discontinuation was numerically lower with Isa-Kd than with Kd across subgroups: 9.0% vs 13.0% (1 prior line) and 15.2% vs 22.1% (>1 prior line). In the 1 prior line of therapy subgroup, 4 patients (5.1%) in the Isa-Kd arm had a TEAE with fatal outcome during the treatment period. In the >1 prior line of therapy subgroup, 6 patients (6.1%) in the Isa-Kd arm and 6 patients (8.8%) in the Kd arm had a TEAE with fatal outcome during the treatment period.
Conclusions: The addition of Isa to Kd improved PFS and depth of response in patients with relapsed MM, regardless of the number of prior lines of therapy, with a manageable safety profile. Results from this subgroup analysis are consistent with the benefit observed in the overall IKEMA study population and further support Isa-Kd as a standard-of-care treatment for patients with relapsed MM irrespective of prior lines of therapy, including those with first relapse.
Funding: Sanofi.
Disclosures
Capra:Janssen: Speakers Bureau; Sanofi: Speakers Bureau; Amgen: Speakers Bureau. Martin:GlaxoSmithKline and Legend Biotech: Consultancy; Legend Biotech: Consultancy; Amgen, Johnson & Johnson / Janssen, Sanofi, and Seattle Genetics: Research Funding. Moreau:AbbVie, Janssen, Celgene, Amgen, and Sanofi: Honoraria. Oriol:GlaxoSmithKline: Consultancy, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; Janssen: Consultancy; Bristol Myers Squibb: Consultancy, Speakers Bureau. Koh:Sanofi Genzyme: Research Funding. Quach:CSL: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role, receipt of free drug for investigator-initiated study , Research Funding; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: receipt of free drug for investigator-initiated study, Research Funding; Antengene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: receipt of free drug for investigator-initiated study, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role , Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role, receipt of free drug for investigator-initiated study , Research Funding. Rawlings:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Tekle:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Macé:Sanofi: Current Employment. Risse:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Spicka:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen-Cilag: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaMar: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.